Communicated By: Dr. Ann Grens, Biology
Localization of Kinetochore-Associated XKCM1 in PtK2 Cells
XKCM1 (Xenopus Kinesin Catastrophe Modulator 1) is a newly identified kinesin that was shown to be essential for the establishment and the maintenance of mitotic spindles by affecting the microtubule dynamics in Xenopus extracts (Desai et al., 1999). In this research we examined the localization of kinetochore-associated XKCM1 in PtK2 cells via immunofluorescence staining of cells treated with the anti-cancer drugs nocodazole and taxol, which disturb the microtubule dynamics. Upon the addition of taxol, microtubule dynamics were suppressed at the plus-ends, which reduced the tension across kinetochores. Nocodazole, on the other hand, destabilized microtubules, producing a pool of tubulin dimers. This treatment decreased the tension across kinetochores by abolishing the microtubule-kinetochore interaction. The length of XKCM1 staining was then measured at kinetochores upon the addition of each drug. In both drug treatments, the tension of kinetochores was decreased or completely lost in some cases, as we had hypothesized. The results of the experiments are in agreement with the idea that microtubule dynamics have a direct effect on XKCM1 in mammalian cells during mitosis.
During the course of mitosis, the newly duplicated chromosomes must be evenly distributed into the two daughter cells. Appropriate segregation and even distribution of the chromosomes is necessary for the life of the cell. Errors during this process lead to several genetic disorders. The mitotic spindle is the major machinery for chromosome segregation during mitosis. It is an array of microtubules and associated molecules that forms between the opposite poles of a eukaryotic cell, and serves to move duplicated chromosomes apart. Major components of the spindle include kinetochores, polar and astral microtubules, and several MAPs (Microtubule Associated Proteins). Microtubule motor proteins constitute an important class of MAPs, which use the energy of ATP hydrolysis to move along microtubules (Sharp et al., 2000). One major class of motor proteins is the KRP family (Kinesin Related Proteins). KRPs are homologous to kinesin, which is a plus end-directed motor. XKCM1 (Xenopus Kinesin Catastrophe Modulator 1) is a newly identified KRP that was shown to be essential for the establishment and the maintenance of mitotic spindles by affecting microtubule dynamics (Shirasu and Walczak, 1999).
Unlike kinesin and many KRPs, XKCM1 is a member of the Kin I (Kinesin Internal) family of KRPs, which do not exhibit conventional motility (Desai et al., 1999). Experiments using immunofluorescence staining show that XKCM1 is found in a cytoplasmic pool as well as attached to the kinetochores, which are complex structures of protiens that form on the opposite sides of the centromere of a mitotic chromasome. It is suggested that XKCM1 in the cytoplasmic pool aids in the establishment and maintenance of microtubule dynamics during mitotic spindle formation. Recent analysis of XKCM1 in Xenopus egg extracts showed that depletion of XKCM1 results in a dramatic increase in microtubule polymerization, which suggests that XKCM1 promotes microtubule destabilization. In support of this idea, it was also shown that purified XKCM1 depolymerizes microtubules in vivo (Smith-Kline and Walczak). Because microtubule polymerization and depolymerization are important in chromosome movement, and XKCM1 is found at kinetochores on chromosomes, it is possible that the depolymerization activity of XKCM1 is necessary for chromosome movement during mitosis.
Chromosome motility is essential for aligning the chromosomes and eventually segregating them to the opposite poles of the spindle. During mitosis, a cell undergoes the stages prophase, metaphase, anaphase, telophase, and finally cytokenisis. In prophase, as the nuclear envelope breaks down, the chromosomes become exposed to the microtubules of the forming spindle. At this stage, the microtubules maintain their dynamic motion of growth and shrinkage as they capture the kinetochore. As the kinetochore on one side of the centromere of a chromosome becomes captured by microtubules, the chromosome is said to be mono-oriented. Mono-orientation, however, is not sufficient for correct segregation of the sister chromatids. In order for the spindle to become in full control of a chromosome, both kinetochores on a chromosome must become attached to microtubules emanating from the opposite spindle poles. At this point, the chromosome becomes bi-oriented. During metaphase, the microtubules maintain their dynamic oscillations as they align the chromosomes along the metaphase plate, which is the plane of the spindle approximately equidistant from the two poles along which the chromosomes are lined up during mitosis (also termed the equator). The separation of sister chromatids marks the beginning of anaphase, which is achieved by the depolymerization of microtubules from their kinetochores with the aid of several motor proteins.
Kinetochores play an active part in the movement of chromosomes to the poles as they become attached to the microtubules of the spindle (Walczak, 2000). During prometaphase, a kinetochore in a mammalian cell will bind 20-30 microtubules. As a chromosome becomes bi-oriented, tension mounts across the kinetochores of that chromosome, stretching the kinetochores and thus increasing the distance between them. The plus ends of kinetochore microtubules remain in a state of dynamic flux, generating the movement of chromosomes until the chromosomes are aligned across the metaphase plate. This requires that both the addition and the loss of tubulin molecules take place while the kinetochore maintains a firm mechanical attachment to the microtubules.
The goal of this research is to determine whether the depolymerization action of XKCM1 is directly required for chromosome movement during mitosis in vivo. Recent analysis of XKCM1 in Xenopus egg extracts showed that depletion of the XKCM1 resulted in a dramatic increase in microtubule polymerization, suggesting that XKCM1 promotes microtubule destabilization (Desai et al., 1999) which raised the question of whether XKCM1 has a similar function in vivo. We hypothesized that the localization and functions of XXCM1 in the mammalian PtK2 cells during mitosis would be similar to that observed in the earlier in vivo studies done on Xenopus egg extracts. We took advantage of the morphological change that Kinetochores undergo due to tension to measure the length of XKCM1 staining at kinetochores in different stages of mitosis, which would reveal the site at which XKCM1 localizes at different mitotic stages. We use two methods to investigate the action of XKCM1, drug treatments and dominant negative experiments.
The goal of drug treatments was to alter the interaction of microtubules with the kinetochores. Nocodazole on the other hand, destabilizes microtubules by binding to tubulin dimers and thus depleting the amount of free dimers. This treatment should also reduce tension across kinetochores by abolishing the microtubule-kinetochore interaction. We then measured the length of XKCM1 staining at kinetochores upon the addition of each drug. Such measurements would allow us to observe the location of XKCM1 during each stage of mitosis.
The idea behind the generation of dominant negative constructs is to generate a version of XKCM1 that will block the function of endogenous XKCM1 specifically at kinetochores. XKCM1 is an 85kDa protein with an N-terminal globular domain, a central catalytic domain, and an alpha- helical C-terminal tail. The globular domain is essential for targeting of XKCM1 to the kinetochore (Smith-Kline and Walczak), the catalytic domain is necessary for depolymerization of microtubules, and the - helical tail aids in dimerization of XKCM1. It was shown previously that the N-terminus of XKCM1 is sufficient to target to kinetochores of spindles assembled in Xenopus egg extracts. Because this construct lacks the catalytic domain, it is unable to depolymerize microtubule resulting in misaligned chromosomes on the spindle. We wanted to construct vectors with sequences coding for only the N-terminus of XKCM1 to allow their expression in a normal cell. This would allow us to see the effect of each domain negative version of XKCM1on both microtubule dynamics and on microtubule-kinetochore interactions.
Materials and Methods
Drug Treatments
PtK2 cells growing in MEM-alpha complete media (fetal bovine serum, L-glutamine, and pen/strep antibiotics) were plated at low concentration on glass coverslips. Cells were allowed to replicate for approximately 72 hours at 37C, 5% CO2 incubator. Drug treatments involved incubation of cells in either taxol or nocodazole for 30 min at 37°C, 5% CO2. Taxol and nocodazole were diluted in DMSO prior to addition to media. Both drugs were applied to cells at final concentrations of 10uM and 10 ug/ml, respectively. DMSO diluted in media was applied to cells and used as a negative control.
Immunofluorescent Staining of Tubulin and XKCM1
Cells were initially rinsed with 1X PBS (NaCl, Tris-Cl pH 7.4). For fixation, PBS was replaced with BRB-80 (PIPES pH 6.8, MgCl2, EGTA), 4% formaldehyde, 5 mM EGTA and left to incubate for 20 min, followed by a wash with a flowing stream of TBS-TX (NaCl, Tris-Cl pH 7.4,Tris Buffer Saline-Triton-X 100). To block nonspecific binding of antibodies, the cells were incubated in blocking buffer (1X TBS-TX, 2% BSA, 0.1% NaN3) for 30 min. To stain for XKCM1 and tubulin, cells were incubated in primary antibody, antiXKCM1 (rabbit antibody) at 5ug/ml followed by a 30 min incubation of the primary antibody against tubulin, DM1-alpha (all antibodies were diluted in 2% BSA, TBS-TX, and stored at 4°C). To visualize the structures under a fluorescent microscope, the cells were incubated in the secondary antibodies goat anti-rabbit FITC and Donkey anti-mouse-Texas Red for 30 min each, respectively. Finally, staining for DNA involved a 5 min incubation of cells in Hoechst diluted in TBS-TX at 1ug/ml. Following each antibody incubation, coverslips were washed with 1XTBS-TX. Coverslips were then mounted on glass slides using antifade mounting media. Cells were viewed under fluorescence microscope. Images were taken on a Nikon E-600 epifluorescence microscope with a Princeton instruments micro Max 1300Y camera. Images were acquired as a Z-step series of 1um steps (4-26 planes) under 100X objective. The z-series was then projected as a maximal projection. All cameras, shutters, a Z-stepper were controlled by Metamorph software.
pEGFPC1-NLS and pEGFPC1-NLS-11BNT
pEGFPC1-NLS is the control vector carrying the sequence for GFP (Green Fluorescent Protein) and NLS (The Nuclear Localization Sequence), which is the sequence necessary for targeting the construct to the nucleus. This is necessary because XKCM1 assembles onto kinetochores prior to nuclear envelope breakdown. To construct each vector, the NLS was PCR amplified from a pCMV/myc/nuc/GFP (purchased from Clontech) using the primers PCMV-F4 5'GGC TTC GAG CGG CCG CAG ATC CAA AAA AG3' and PCMV-R1 5'CCC GGT ACC GGC CCC ATT CAG ATC CTC3'. The PCR product was digested with the restriction digest enzymes XhoI and KpnI and cloned into the parental vectors pEGFPC1 and pEGFPC1-11BNT to generate pEGFPC1-NLS and pEGFPC1-NLS-NT (Figure 1).
Generation of constructs
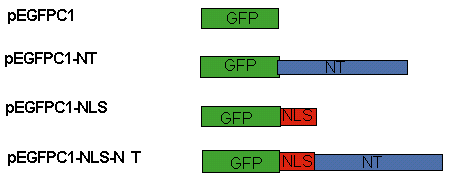
Figure 1: Cloning Strategy
pEGFPC1 and pEGFPC1-NT are the parent vectors. Upon the insertion of NLS into each parent vector, the pEGFPC1-NLS and pEGFPC1-NLS-NT are produced. pEGFPC1-NLS is the control, while pEGFPC1-NLS-NT is the vector carrying the N-terminus of the XKCM1.
Results
To determine the localization of the kinetochore-associated XKCM1 and the structure of microtubules as a consequence of the addition of taxol and nocodazole, the cells were fixed and stained immediately following the drug treatments. PtK2 cells were selected for this experiment since they have large chromosomes that allow easy visualization of kinetochores. Cells were examined for the morphology of the mitotic spindle as well as the distribution of XKCM1 staining in the cell. The inner distance between opposite kinetochores was measured by determining the distance between the center of the XKCM1 staining at each kinetochore.
The DMSO-treated control cells displayed features of a normal spindle. In prophase cells, where chromosomes are not yet attached to the mitotic spindle, XKCM1 was observed in a cytoplasmic pool and could also be seen at the kinetochores of the chromosomes (Figure 2A). For the DMSO-treated cells at prophase, the average inter-kinetochore distance was 1.3 + 0.2um (Table I). Most XKCM1 observed were in the structure of two closely positioned dots. Prometaphase is the mitotic stage where the microtubule dynamics, along with several motor proteins are in the process of aligning the chromosomes along the metaphase plate. The average inter-kinetochore distance during this stage was measured to be 2.7 + 0.5 um (Table I). The structure of XKCM1 was a dumbbell shape (data not shown). Cells viewed at metaphase display normal chromosome alignment at the metaphase plate as well as a normal spindle structure (Figure 3B). The structure of XKCM1 was similar to that in the prometaphase, however, in a less disperse alignment, as expected (Figure 3A).
The nocodazole-treated cells appeared abnormal. Instead of the long microtubules of a normal spindle, a soluble pool of tubulin dimers was observed. The chromosomes showed a dispersed arrangement in the region of the metaphase plate. XKCM1 was found in the cytoplasm and also localized to the kinetochores. Inner kinetochore distance in nocodazole treated cells was similar to that of DMSO-treated cells at prophase (Table I). XKCM1 structure appeared as two closely positioned dots, similar to that of the control cells at prophase (Figure 4A).
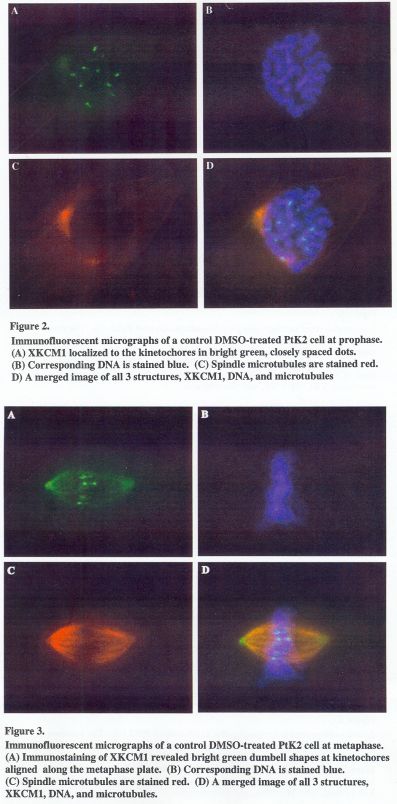
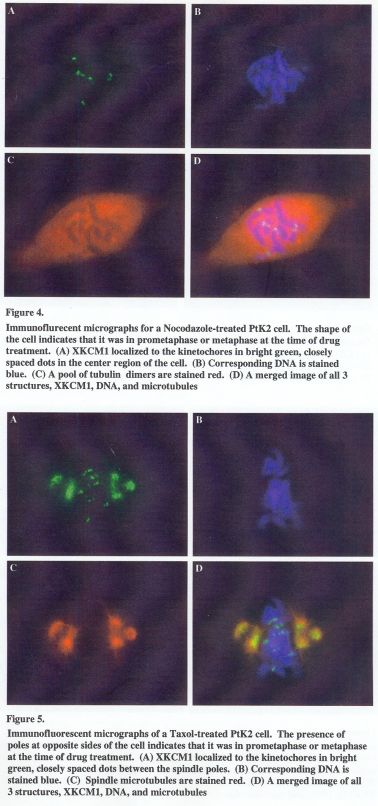
Table 1: Drug Treatments and Inter-Kinetochore Distance
This table shows stages of mitosis at which cells were undergoing as they were fixed and stained, the type of drug treatment, the average inter-kinetochore staining in 2 m, and the standard deviation.
| Kinetichoret sample | Avg interkinetochore distance | SD | |
| Stage / Drug | size | (um) | (um) |
| Metaphase/Control | 18 | 2.7 | 0.5 |
| Prophase/Control | 13 | 1.3 | 0.2 |
| Metaphase/Noc | 11 | 1.1 | 0.2 |
| Metaphase/Tax | 10 | 1.1 | 0.1 |
Table 2: P-values Obtained by the Student's T-Test
This table compares the similarity of the measured inter-kinetochore distance between the different mitotic stages and drug treatments by showing the p value for each of the two groups. A p-value of 3 0.05 represents a statistically significant similarity between two groups.
| Stage / Drug | vs. | Stage / Drug | p value |
| promet & met/DMSO | pro/DMSO | 0.0000 | |
| promet & met/DMSO | met/Noc | 0.0000 | |
| promet & met/DMSO | met/Tax | 0.0000 | |
| pro/DMSO | met/Noc | 0.1600 | |
| pro/DMSO | met/Tax | 0.2735 | |
| met/Noc | met/Tax | 0.2462 |
The structure of the spindle was disrupted upon the addition of taxol. Staining of microtubules showed a large density of astral microtubules at the poles and very low density towards the metaphase plate region. XKCM1 localization was mostly restricted to the center portion the asters (Figure 5A). The kinetochore XKCM1 remained visible and appeared as two closely spaced spots. The inter-kinetochore distance was measured and found to be 1.1 + 0.1 2 m (Table 1), which is almost identical to that of a kinetochore of the nocodazole treated cells. However, when compared to that of the nocodazole treated cells, XKCM1 appears much less compact in structure.
Cloning Experiments
Previous studies on XKCM1 from Xenopus egg extracts showed that the N-terminus of XKCM1 was sufficient for targeting to the proteins to the kinetochores. We wanted to see whether XKCM1 behaves in the same way in vivo, therefore we constructed two different constructs. The two constructs are pEGFPC1-NLS and pEGFPC1-NLS-NT. pEFPC1 in the control vector and pEGFP-NLS-NT carries the nucleotide sequence coding for the N-terminus domain of XKCM1(Figure 1). Unfortunately, after constructing the vectors, sequencing reactions revealed that we had not actually obtained the correct vector, which is thought to be due to contamination. However, we are currently in the process of rebuilding the constructs. After the constructs are obtained, transfection of each vector in PtK2 cells will be carried out to determine the requirement for XKCM1 targeting to kinetochores.
Discussion
Drug Treatment Experiments
By comparing the inter-kinetochore distance of the control cells at prometa/metaphase with those treated with nocodazole and taxol, it is apparent that inter-kinetochore distance is twice as long when the microtubules of the spindle are attached to the kinetochores. These results support our predictions since nocodazole has a destabilizing effect on microtubules; it thus reduces the tension across kinetochores, decreasing the distance between a pair of kinetochores of a chromosome. The spindles of cells treated with nocodazole appeared as a pool of tubulin subunits, and no microtubules were observed. Taxol, on the other hand, appeared to only decrease the amount of microtubules-kinetochore attachments. Its effect on XKCM1 was similar to that of nocodazole, which is evident by the measurements (Table I).
A student's t-test, also known as a W.S. Gossett test, is a statistical test that is used to compare the means of two populations and to determine whether or not the means of the sample populations are significantly different. This test was used to further analyze the results for each treatment (Table II). According to the t-test results, the length of XKCM1 staining in prometaphase and metaphase in control cells showed no similarity in nocodazole or taxol-treated cells. These data further support our rational concerning the inter-kinetochore tension in relation to microtubule dynamics.
Dominant Negative Experiments: Cloning Strategy and Future Research
The N-terminus of XKCM1 was shown to be sufficient for targeting the protein to the kinetochores in the previous studies on XKCM1 from Xenopus egg extracts. The dominant negative experiments would allow us to determine if the function is similar in vivo. The transfection of pEGFPC1-NLS and pEGFPC1-NLS-NT in PtK2 cells would allow us to examine whether the amino terminus is indeed sufficient for targeting to the kinetochore in vivo.
It is unfortunate that the vectors pEGFPC1-NLS and pEGFPC1-NLS-NT are not yet constructed. However, the dominant negative experiments would allow us to explore the function of each of the XKCM1 domains, its involvement in the chromosome positioning and progression of mitosis. Further research in this area will involve the production of the constructs pEGFPC1-NLS and pEGFPC1-NLS-NT. In addition to those constructs, the constructs pEGFPC1-NLS-NTCT, which carry the nucleotide sequences for N-terminus and carboxy terminus of XKCM1, and pEGFPC1-NLS-NTCC, which carry the n sequences for N-terminus and the coil-coil domain of XKCM1 can be prepared. By expressing those constructs in normal PtK2 cells, large knowledge of the function of XKCM1 in vivo can be revealed.
For future research in the area of immunofluorescence, the use of a structural marker for kinetochores would be an extremely useful tool. It would allow us to clearly visualize the interactions between XKCM1 and kinetochores, as well as the kinetochore-microtubule targeting. The immunofluorescent staining of drug-treated cells, along with the dominant negative experiments, work hand-in-hand to clarify the function of XKCM1 in cells and its importance during the course of mitosis.
- Desai, A., Verma, S., Mitchison, T. J., and Walczak, C. E. (1999) Kin I Kinesins are Microtubule-Destabilizing Enzymes. Cell 96, 69-78.
- Smith-Kline, S and , C. E. XKCM1 Depolyemerized Microtubules in Cells (in Preparation)
- Sharp, D. J., Rogers, G. C., and Scholey, J. M. (2000) Microtubule Motors in Mitosis. Nature 407, 41-47.
- Shirasu, M., Yonetani, A, and Walczak, C.E. (1999) Microtubule Dynamics in Xenopus Egg Extracts. Microscopy Research and Technique 44, 435-445.
- C. E. (2000) Microtubule Dynamics and Tubulin Interacting Proteins. Current Opinion in Cell Biology 12, 52-56.


